

Major debilitating baseline mental health disorders (e.g.Conditions or circumstances that would interfere with follow-up and outcome assessment, as determined by the principal investigator or delegate. Subject or LAR provided informed consent for the Acute Blood Biomarker Branch and the Follow-up Branchįollow up Branch Subject Exclusion Criteria:ġ.Enrollment in Acute Blood Biomarker Branch.BUSINESS ADDRESS: Abbott Laboratories Inc. Standard of care head CT scan procedures not completed prior to Emergency Department (ED) discharge.įollow-up Branch Subject Inclusion Criteria: ITEM NUMBER: 605-6b i-STAT 1 Analyzer (Hand-Held Battery Operated).Presented with penetrating head trauma or spinal cord injury (American Spinal Injury Association score of C or worse) at the enrolling facility.Time of injury is unknown and cannot be estimated.Primary diagnosis at the enrolling facility of ischemic or hemorrhagic stroke.
ABBOTT ISTAT TRIAL
Current (on-going) enrollment in a therapeutic or interventional clinical trial (drug or device).Previous enrollment in this study, CS-2018-0009.

Focal neurological deficits that may or may not be transientĪcute Blood Biomarker Branch Subject Exclusion Criteria:.Any alteration of mental state at the time of the injury.Any loss of memory for events immediately before or after the injury.Subject has a computed tomography (CT) scan of the head with all sequences (bone and soft tissue) ordered as part of standard of care at the enrolling facility, or are transferred to the enrolling facility with a head CT scan sent from the originating facility.Īs a result of this head injury, the subject has sustained a traumatically induced physiological disruption of brain function, as manifested by at least one of the following:.Subject presented to a health care facility or emergency department with a suspected traumatic brain injury resulting from an insult to the head by an external force within 12 hours of the injury.Subject or Legally Authorized Representative (LAR) provided informed consent for the Acute Blood Biomarker Branch (waiver of consent may be acceptable, per IRB).Why Should I Register and Submit Results?Īcute Blood Biomarker Branch Subject Inclusion Criteria:.Alternatively, multiple analysers can transmit data to a central workstation. Up to 50 results are automatically stored within the analyser and these can be printed out at any time by directly linking the analyser to a small Infrared printer. Samples are processed immediately and provide lab-quality results in 2 minutes making the i-STAT quick and easy to use.Ĭalibration is automatic and contained within the test cartridges simplifying quality control procedures. Texans are encouraged to report damage to homes and businesses using the Individual State of Texas Assessment Tool (iSTAT).
ABBOTT ISTAT VERIFICATION
Only a few drops of whole blood are required for testing, between 65 and 95 µl. Tricontrols is a comprehensive set of aqueous control and calibration verification materials that combine the ability to assay hematocrit, blood gases. At the direction of Governor Abbott, TDEM also activated five additional Texas A&M Task Force 1 swiftwater boat squads and a floodwater boat squad to support local response efforts. There are a number of cartridges testing different combinations of analytes therefore eliminating the need for different analysers.
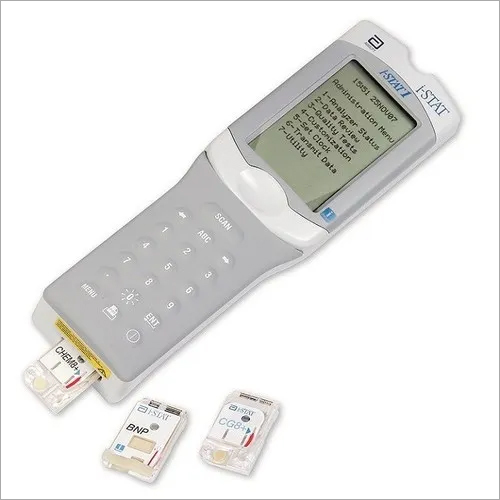
ABBOTT ISTAT PORTABLE
The i-STAT Portable Clinical Analyser is a true point of care analyser designed to be used at the patients bedside for critical care tests such as blood gases, electrolytes, metabolytes and coagulation. The analyzer stores up to 50 patient records and permits on-screen viewing of test results as well as transmission of records to a data management system using infrared signals. The i-STAT portable clinical Analyzer (PCA) is used in conjunction with disposable cartridges for determination of a variety of parameters in whole blood. The components used to make the Abbott i-STAT 300 Handheld ABG Blood Analyzer are acquired from the most reputable and authentic sources following thorough market research. DESCRIPTION This portable clinical analyzer is used in conjunction with disposable cartridges for determination of a variety of parameters in whole blood. All Abbott i-STAT 300 Handheld ABG Blood Analyzers are made using high-quality materials and innovative procedures, ensuring that they meet the industrys high standards.


 0 kommentar(er)
0 kommentar(er)
